This article introduces you to some basic knowledge of supercapacitors.
1. Concept
what is a supercapacitor
A supercapacitor or ultracapacitor is a new energy storage device between the traditional capacitor and the rechargeable battery, which has the characteristics of fast charging and discharging of the capacitor, and at the same time has the energy storage characteristics of the battery.
This is an energy storage device that bridges the gap between traditional capacitors and batteries. It is designed to store and release large amounts of electrical energy quickly. This type of capacitor has a high capacity to store the energy charge and has a very high value of capacitance than the normal capacitor (up to 2KF).
The high surface area of the activated carbon electrodes allows for a larger number of ions to be stored, resulting in a higher energy storage capacity compared to traditional capacitors.
Although supercapacitors have higher power density and faster charge/discharge rates than batteries, they typically have lower energy density. This means they can store less energy per unit of mass or volume compared to batteries. However, supercapacitors have a longer cycle life and can withstand a higher number of charge/discharge cycles without significant degradation.
2. Principle
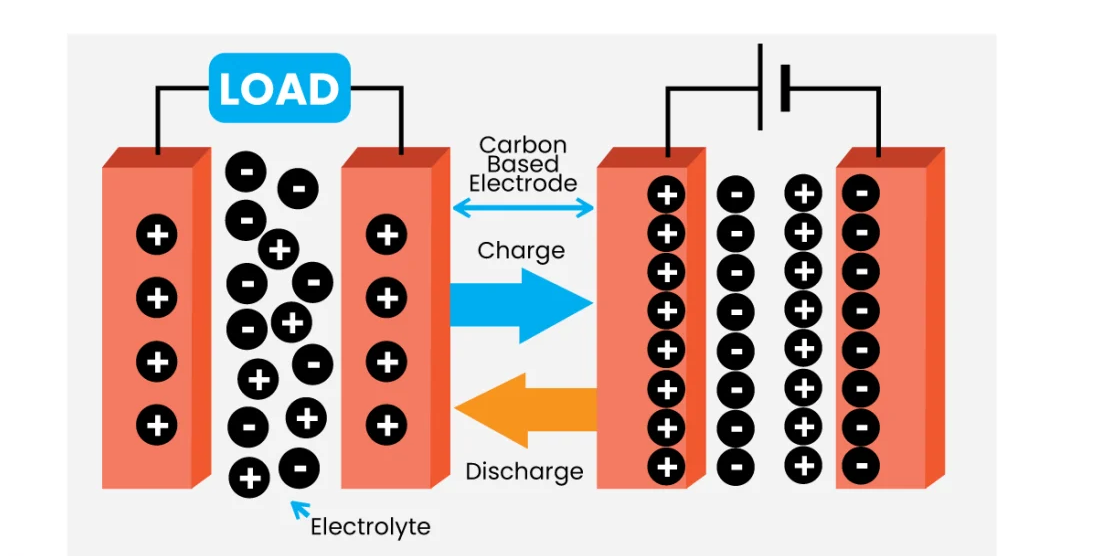
A supercapacitor is a new type of component that stores energy through a two-layer interface formed between an electrode and an electrolyte. When the electrode is in contact with the electrolyte, due to the effects of Coulomb force, intermolecular force and interatomic force, the solid-liquid interface exhibits a stable double-layer charge with an opposite sign, which is called an interface double layer. The electric double-layer supercapacitor was regarded as two inactive porous plates suspended in an electrolyte, and the voltage was applied to two plates. An electric double-layer capacitor is created on the surface of the two electrodes when the potential provided to the positive electrode plate attracts negative ions in the electrolyte and the potential applied to the negative electrode plate attracts positive ions. The electric double-layer capacitor can be classified into a carbon electrode double-layer supercapacitor, a metal oxide electrode supercapacitor, and an organic polymer electrode supercapacitor depending on the electrode material.
Image source- https://sinovoltaics.com/
The working of a supercapacitor involves the principles of electrostatic charge separation and the behavior of electrolytes. Here’s a step-by-step explanation of how a supercapacitor operates:
- Electrodes: A supercapacitor consists of two electrodes, typically made of a porous material with a high surface area, such as activated carbon. These electrodes provide a large surface area for electrostatic charge storage.
- Electrolyte: The electrodes are immersed in an electrolyte solution, which acts as a medium for ion transport. The electrolyte can be an aqueous solution or an organic solvent with dissolved salts. The choice of electrolyte depends on the specific type of supercapacitor.
- Charge separation: When a voltage is applied across the electrodes, one electrode becomes positively charged (anode) while the other becomes negatively charged (cathode). This voltage causes the electrolyte ions to migrate and accumulate at the electrode-electrolyte interface.
- Electric double layer formation: At each electrode-electrolyte interface, an electric double layer is formed. The double layer consists of two regions: the Helmholtz layer and the diffuse layer. In the Helmholtz layer, the ions are tightly bound to the electrode surface, while in the diffuse layer, the ions are more loosely associated.
- Energy storage: The energy storage mechanism in a supercapacitor involves the physical adsorption of ions at the electrode-electrolyte interface. When the voltage is applied, positive ions from the electrolyte are attracted to the negatively charged electrode (cathode) and accumulate in the double layer, while negative ions move towards the positively charged electrode (anode). This charge separation process results in the storage of electrical energy.
- Discharge: When the supercapacitor is connected to a load or an electrical circuit, the stored energy can be discharged. The accumulated ions are released from the electrodes, and the charge imbalance is neutralized. As a result, electrical current flows through the circuit, and the supercapacitor gradually loses its charge.
- Charge and discharge cycles: Supercapacitors are known for their ability to undergo a large number of charge and discharge cycles without significant degradation. This is because the energy storage mechanism relies on physical processes rather than chemical reactions, as seen in batteries. The absence of chemical reactions reduces wear and tear on the electrodes, resulting in a longer cycle life.
By utilizing the principles of charge separation, electric double-layer formation, and ion adsorption, supercapacitors provide high power density, rapid charge/discharge capabilities, and long cycle life, making them suitable for various applications requiring quick bursts of energy or frequent energy cycling
3. Features of supercapacitor
In comparison to batteries and conventional physical capacitors, supercapacitors’ properties are primarily reflected in:
(1) High power density. It can achieve 102–104 W/kg, which is significantly more than the battery’s power density level.
(2) Long cycle life. After a high-speed deep charge and discharge cycle of 500,000 to 1 million cycles in a few seconds, the characteristics of the supercapacitor change little, and the capacity and internal resistance are only reduced by 10% to 20%.
(3) The working temperature limit is wide. Since the adsorption and desorption rates of ions in the supercapacitor do not change much at low temperatures, their capacity changes are much smaller than those of batteries. Commercial supercapacitors have an operating temperature range of -40°C to +80°C.
(4) Maintenance free. The supercapacitor has high charging and discharging efficiency, has a certain tolerance to overcharge and over-discharge, and can stably charge and discharge repeatedly, and theoretically, maintenance is not required.
(5) Environment friendly. Supercapacitors do not use heavy metals and other harmful chemicals in the production process and have a long life span, so they are a new type of green power source.
4. Classification
Types of supercapacitors
For supercapacitors, there are different classification methods depending on the content.
First, supercapacitors can be classified into two groups based on various energy storage mechanisms:
- Faraday quasi capacitors
- electric double-layer capacitors.
The electric double-layer capacitor, among them, primarily produces adsorption energy by adsorbing on the electrode’s surface with pure electrostatic charge. In order to achieve energy storage and conversion, Faraday quasi-capacitors primarily generate Faraday quasi-capacitance through reversible redox reactions on the surface and near the surface of Faraday quasi-capacitor active electrode materials (such as transition metal oxides and high molecular polymers).
Secondly, depending on the type of electrolyte, it can be divided into two categories:
- water-based supercapacitors
- organic supercapacitors.
In addition, depending on whether the types of active materials are the same, they can be classified into symmetric supercapacitors and asymmetric supercapacitors.
symmetric supercapacitors and asymmetric supercapacitors.
- Symmetric Supercapacitors: Symmetric supercapacitors, also known as double-layer capacitors (EDLCs), have identical electrode materials and similar capacitance values for both the positive and negative electrodes. They rely on the electrostatic charge separation principle, where energy is stored through the formation of electric double layers at the electrode-electrolyte interface. Symmetric supercapacitors offer balanced performance in terms of power density and energy density, making them suitable for applications that require high power delivery and moderate energy storage.
- Asymmetric Supercapacitors: Asymmetric supercapacitors have different electrode materials and capacitance values for the positive and negative electrodes. The asymmetry is intentional and results in distinct electrochemical behavior at each electrode. Typically, one electrode has a higher specific surface area and mainly contributes to double-layer capacitance, while the other electrode incorporates a pseudocapacitive or redox-active material, providing additional capacitance through faradaic reactions. Asymmetric supercapacitors aim to combine the benefits of both electric double-layer capacitance and pseudocapacitance, offering enhanced energy density while maintaining a good power density.
The design of asymmetric supercapacitors allows for a broader range of electrochemical reactions and a higher overall capacitance, which translates into increased energy storage capacity. By utilizing different electrode materials with complementary characteristics, asymmetric supercapacitors can achieve a balance between high power delivery and improved energy storage. This makes them suitable for applications that require a longer discharge time or higher energy output, such as renewable energy systems, electric vehicles, and portable electronics.
It’s important to note that asymmetric supercapacitors can also be classified as a type of hybrid supercapacitor, as they combine the features of electric double-layer capacitance and pseudocapacitance in a single device.
Secondly, according to the state form of the electrolyte, the supercapacitor can be divided into two categories: a solid electrolyte supercapacitor and a liquid electrolyte supercapacitor.
solid electrolyte supercapacitor and a liquid electrolyte supercapacitor.
- Solid Electrolyte Supercapacitor: In a solid electrolyte supercapacitor, the traditional liquid electrolyte is replaced with a solid-state electrolyte. The solid-state electrolyte can be a solid polymer, ceramic, or composite material that exhibits ionic conductivity. Solid electrolytes offer advantages such as improved safety, better stability, and the potential for higher operating voltages. They are less prone to leakage, do not require a separator, and can withstand higher temperatures. Solid electrolyte supercapacitors are still an area of active research and development, aiming to enhance their performance and energy density.
- Liquid Electrolyte Supercapacitor: In a liquid electrolyte supercapacitor, the electrolyte is in a liquid or gel form, typically consisting of an organic solvent with dissolved salts. The liquid electrolyte provides a conductive medium for the ions to move between the electrodes during charge and discharge cycles. Liquid electrolyte supercapacitors have been extensively studied and commercialized, offering high power density and fast charge/discharge rates. However, liquid electrolytes may have limitations regarding safety, as they can be flammable and volatile. They also require the use of a separator to prevent the electrodes from coming into direct contact.
Both solid electrolyte and liquid electrolyte supercapacitors have their advantages and limitations. Solid electrolyte supercapacitors have the potential for improved safety and stability, while liquid electrolyte supercapacitors currently offer higher power density. The choice between the two depends on the specific application requirements, considering factors such as energy density, power density, safety concerns, operating conditions, and cost. Ongoing research aims to further optimize and advance both types of supercapacitors to meet the evolving demands of various industries
5. Main parameters of supercapacitor
1) Lifetime: When the internal resistance of the supercapacitor increases, the capacity reduction j is within the specified parameter range, and its effective use time can be extended, generally related to its characteristics as specified in Article 4. The impact on life is active dryness, increased internal resistance, and the ability to store electrical energy drops to 63.2%.
2) Voltage: The supercapacitor has a recommended voltage and a recommended operating voltage. If the used voltage is higher than the recommended voltage, the life of the capacitor will be shortened, but the capacitor can work continuously for a long time under the high voltage state, and the activated carbon inside the capacitor will decompose to form a gas. It is advantageous to store electrical energy, but it can’t exceed 1.3 times the recommended voltage, otherwise, the supercapacitor will be damaged due to the high voltage.
3) Temperature: The normal operating temperature of the supercapacitor is -40 to 70 °C. Temperature and voltage are important factors influencing the life of supercapacitors. For every 5 °C increase in temperature, the life of the capacitor will drop by 10%. At low temperatures, increasing the operating voltage of the capacitor, the internal resistance of the capacitor does not rise, and the efficiency of use of the capacitor can be improved.
4) Discharge: The internal resistance of the capacitor plays a significant role in the pulse charging technology, whereas the capacity plays a significant role in the tiny current discharge.
5) Charging: There are many ways to charge the capacitor, such as constant current charging, constant voltage charging, and pulse charging. During the charging process, connecting a resistor in the capacitor circuit will reduce the charging current and improve the battery life.
Uses of supercapacitor
Automobiles, Elevators, Trains, Cranes, and other areas which required short-term energy storage and rapid charge-discharged cycle.
Also read
-
What is Zero crossing detector circuit
-
Difference between 12-0-12 and 0-12 transformer
-
why ac current passes through capacitor but dc can’t
-
Electronics Multiple choice question MCQ